FDA Approves First Rapid COVID-19 At-Home Testing Kit
Since COVID-19 has made its way into the United States, if you needed to get tested, you had to go into a medical office of some sort. That will be changing soon.
The U.S. Food and Drug Administration has now issued an emergency use authorization for the first self-test for COVID-19 that provides rapid results at home.
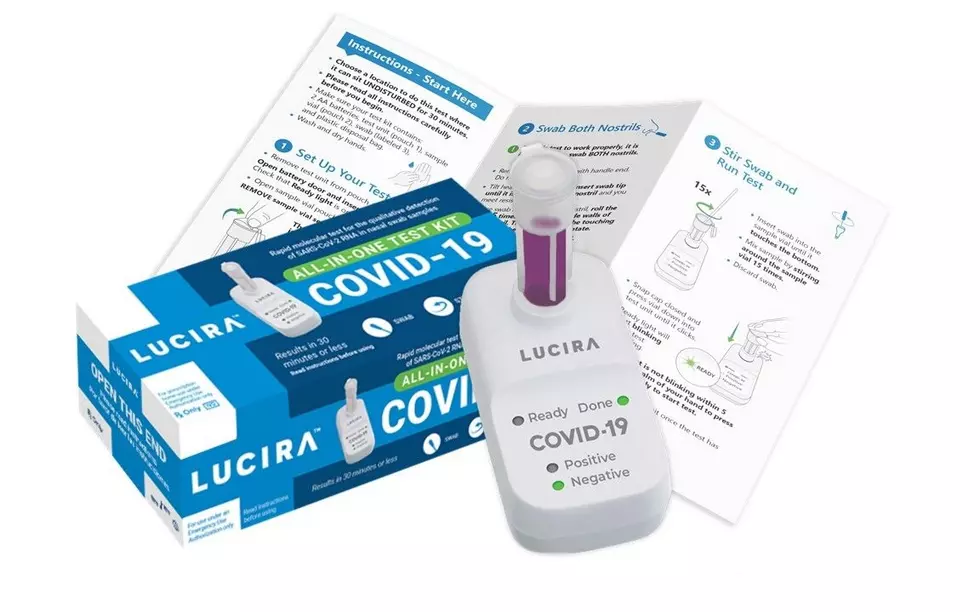
The Lucira COVID-19 All-In-One Test Kit is described as a molecular single-use test that can be purchased with a prescription for self-diagnosis of the coronavirus. The FDA says it can return results in 30 minutes.
"While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home," FDA Commissioner Dr. Stephen Hahn said in a statement.
This new at-home test, which uses self-collected nasal swab samples, is for patients 14 and older with suspected COVID-19 and people under 13 when performed by a health care provider.
"This new testing option is an important diagnostic advancement to address the pandemic and reduce the public burden of disease transmission," Hahn added.
The new self-testing kit comes with a sterile swab, a sample vial, a test unit, batteries and a plastic disposal bag.
The sample collected on the nasal swab goes into the vial which then enters the test unit where it is analyzed. The results are then displayed on the unit by a color change in the LED indicators.
This news comes on the heels of Monday's announcement by US-based pharmaceutical company, Moderna that its experimental COVID-19 vaccine is 94.5 percent effective in preventing the illness.
Five Tips for Cold and Flu Season
More From Classic Rock 105.1